
Clinical, organizational and economic analysis of high-sensitivity cardiac troponin testing in the emergency department
Introduction
The measurement of cardiac troponins (cTns) has become the mainstay for the diagnosis of acute coronary syndrome (ACS), since the assessment of either cardiac troponin T (cTnT) or cardiac troponin I (cTnI) has now outperformed whatever other available biomarker, to be used alone or in combination with cTns (1). This paradigm shift has been strongly supported by recent introduction in clinical practice of the so-called high-sensitivity (HS) immunoassays, which have allowed to considerably enhance the functional and diagnostic sensitivity of the former “contemporary-sensitive” (CS) techniques, thus allowing a more efficient and faster patient management in short stay units such as the emergency department (ED) (2).
Regardless of the many diagnostic protocols that have been developed so far for improving the clinical efficiency of cTns testing in diagnosing ACS, and entailing baseline and 1-, 2-, 3- or 6-hour serial testing, using either absolute or relative increases from the baseline (3), the clinical advantage of HS-cTns immunoassays for rapid rule-out of ACS has been clearly demonstrated by many studies, especially those based on protocols or algorithms entailing a diagnostic cut-off lower than the conventional 99th percentile of the upper reference limit (URL) (4-6). Nevertheless, the potential economic and organizational benefits of using these innovative techniques in the ED remain mostly speculative, since no studies have been published so far about this important aspect to the best of our knowledge (7).
Therefore, the present study was aimed to compare the combined clinical, organizational and economic advantages of replacing a CS-cTnI technique with a new commercially available HS-cTnI immunoassay for diagnostics of patients admitted to the ED with suspected ACS.
Methods
The study population consisted of 288 consecutive patients [mean age 63 years (range, 50–77 years), 169 males and 119 females], who were admitted to the ED of the University Hospital of Parma (Italy) for suspected ACS over a 3-month period. Symptoms of suspected ACS included chest pain of non-traumatic origin, both radiated or non-radiated to arms, neck or jaws, and both associated or non-associated with diaphoresis, nausea/vomiting, palpitations or syncope/pre-syncope. The University Hospital of Parma is a 1,150-bed teaching hospital, serving a population of about 435,000 inhabitants, and is the only general hospital in the town of Parma. The facility is also a level 2 trauma center and a referral center for stroke and ACS.
The cTnI was measured with two commercial immunoassays, characterized by different analytical and functional sensitivities. The former was a CS-cTnI immunoassay (AccuTnI+3; Beckman Coulter Inc., Brea CA, USA) used on the Beckman Coulter’s UniCel DxI 800 analyser, and characterized by functional sensitivity and 99th percentile URL of 37 and 50 ng/L, respectively (8). The latter method was instead a HS-cTnI immunoassay (Abbott Architect STAT HS cTnI), used on the Abbott Architect i2000SR/i1000SR immunoanalyzers, and characterized by functional sensitivity and 99th percentile URL of 5.6 and 19.3 ng/L, respectively (9). The same diagnostic strategy was used for analysing the clinical, organizational and financial endpoints of this study. Therefore, serial testing was based on a first measurement upon patient admission to the ED, whilst a second measurement was performed 3 hours afterwards (i.e., 0–3 hours strategy), ordered according to results of the first cTnI testing or to clinical judgement.
According to recent evidence, the diagnostic threshold of cTnI was defined as the functional sensitivity of either assay (i.e., 37 and 5.6 ng/L for CS-cTnI and HS-cTnI immunoassays, respectively). According to conventional definition, the functional sensitivity of cTns immunoassays corresponds to the cTn value with ≤10% imprecision. Rapid rule-out of ACS was hence established in all patients with cTnI results below the functional sensitivity of both immunoassays (10). All routine CS-cTnI measurements were made available to the emergency physicians within the recommended turnaround time (i.e., <1 hour). The ED stay was then calculated as 1 hour for patients that would have been discharged according to the first negative cTnI measurement, and 4 hours for those who would have been discharged after a second negative cTnI measurement in samples collected 3 hours after ED admission. According to our local protocol for evaluation of patients with suspected ACS, electrocardiogram (ECG) and collection of blood samples are performed almost simultaneously. The results of the ECG are then rapidly interpreted by the emergency physician. The final diagnosis of ACS was made according to the criteria of the European Society of Cardiology (ESC), as described elsewhere (11). A 30-day follow-up was then established to identify possible cases of ACS which could not be diagnosed upon the first admission. Medical records were hence systematically reviewed by two different emergency physicians, to confirm the original diagnosis according to the ESC criteria.
The HS-cTnI immunoassay was performed using residual plasma, which was promptly frozen after routine CS-cTnI testing had been completed, and then thawed for being reanalyzed with the HS immunoassay. As such, the results of the HS-cTnI immunoassay were unavailable to the emergency physicians, so that the clinical management in the ED was only dependent upon results of the routine CS-cTnI. The medical records of all patients were then re-evaluated by two different emergency physicians after replacing data of the CS-cTnI assay with those of the HS-cTnI technique, in order to identify possible changes of patient management in the ED according to results of the more sensitive test. The total cost of patient management in the ED was estimated according to standardized data reported by Foley et al. (i.e., 66€ for 1 hour of patient stay) (12).
Results were shown as mean and standard deviation (SD). Continuous variables were compared with Mann-Whitney U test, whereas proportions were analyzed using Chi-square test with Yates’ correction, using GraphPad Software (GraphPad Software, Inc., La Jolla, CA, USA).
The study was performed in accordance with the Declaration of Helsinki, under the terms of relevant local legislation. Since the HS-cTnI immunoassay was performed using residual material after routine CS-cTnI testing had been completed, and HS-cTnI test results were not reported to both the emergency physician and to the patient, informed consent was deemed unnecessary.
Results
The results of cTnI measurements performed with both immunoassays in samples collected at the time of ED admission are shown in Table 1. Overall, 198 patients (68.7%) were negative with both methods, 40 (14.0%) were positive with both methods, 1 (0.3%) was only positive with the CS-cTnI assay, whereas the remaining 49 (17.0%) were only positive with HS-cTnI (overall observed agreement, 82.6%).
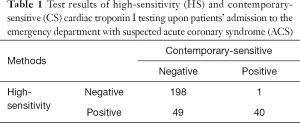
Full table
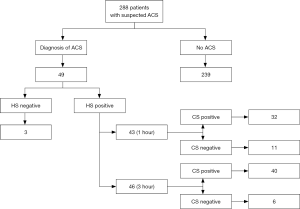
Throughout the follow-up period, 49/288 patients (17%) were finally diagnosed with ACS, 3 with ST-elevation myocardial infarction (STEMI) and 46 with non-ST-elevation myocardial infarction (NSTEMI). Forty of these 49 patients were positive with both methods, 3 were found to be negative using both assays and 6 remained negative up to 3 hours with CS-cTnI but were positive with HS-cTnI. In 43 patients ACS was suddenly identified after the first measurement with HS-cTnI, and 3 additional patients were finally diagnosed with ACS 3 hours thereafter. As regards CS-cTnI, 32 patients were immediately diagnosed with ACS after the first measurement, whereas ACS could be diagnosed in 8 more patients after the 3-hour measurement (Figure 1). In the remaining 6 patients, who tested positive with HS-cTnI but still had the 3-hour sample negative with CS-cTnI, ACS was diagnosed 6 hours later or afterwards, during hospitalization for reasons other than myocardial ischemia. Overall, the HS-cTnI immunoassay thus allowed to rapidly identifying 22% (11/49) and 12% (6/49) more ACSs within 1 and 4 hours, respectively, than using CS-cTnI. On the other hand, 27/288 patients (9.4%) displaying results of HS-cTnI suggestive for ACS at the 3-hour time point, but remaining negative with CS-cTnI, would have been probably hospitalized for performing additional diagnostic investigations according to HS-cTnI data. Interestingly, one of these patients was discharged based on negative results of CS-cTnI, but was then readmitted within 30 days with a diagnosis of ACS. The diagnostic performance of both the HS-cTnI and CS-cTnI immunoassays at admission and 3 hours afterwards is shown in Table 2.

Full table
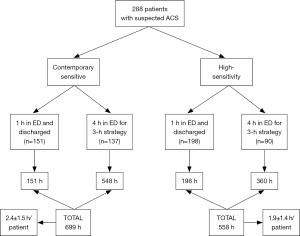
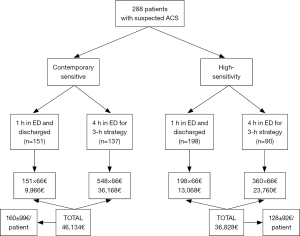
The patient management defined according to results obtained with the two methods is shown in Figure 2. Overall, ACS could be rapidly ruled out (i.e., within 1 hour) in a larger number of patients using HS-cTnI compared to the use of CS-cTnI (68.7% versus 52.4%; P<0.001). Accordingly, the overall stay in the ED for ACS diagnostics was found to be nearly 20% shorter using HS-cTnI than with CS-cTnI (558 versus 699 hours; P<0.001). This was actually mirrored by a lower mean patient stay in the ED using HS-cTnI than with CS-cTnI (1.9±1.4 versus 2.4±1.5 patients/hour; P<0.001). Identical results were obtained by calculating the predictable ED cost of managing patients with results of either method, wherein the mean cost for patient was 128±92€ using HS-cTnI compared to 160±99€ with CS-cTnI (–20%; P<0.001) (Figure 3).
Discussion
In agreement with previous data (6,13), we found that the HS-cTnI immunoassay was characterized by improved efficiency for early rule-out of ACS compared to a conventional CS-cTnI technique (Table 2), thus allowing very early identification of ACS in 11/288 patients (3.8%) in whom the results of CS-cTnI was non-diagnostic at ED admission. The efficiency for ruling out ACS remained higher for HS-cTnI even at the 3-hour sampling time, although the negative predictive value of CS-cTnI tended to approximate that of HS-cTnI (i.e., 0.99 versus 0.96). In support of previous data, we also found that the positive predictive value of CS-cTnI was always better than that of HS-cTnI, both in samples collected at patient admission (0.74 versus 0.48) and 3 hours afterwards (0.98 versus 0.61). The improved efficiency for early diagnosing ACS of HS-cTnI is clearly attributable to its lower functional sensitivity (i.e., 5.6 ng/L), thus allowing to more reliably identifying minor but highly ACS-suggestive increases of cTnI during short serial testing, which may instead be virtually unappreciable when using the higher diagnostic threshold (i.e., 37 ng/L) characterizing our routine CS-cTnI technique.
At variance with previous clinical investigations, however, we firstly showed that the use of HS-cTnI may be really cost-effective in the ED. According to our analysis, we estimated that the time needed for urgent ACS diagnostics may be shortened by approximately 20% when cTnI testing is carried out with a HS technique, thus also having a potentially favourable impact on decreasing ED overcrowding. Recent statistics attests that the many patients admitted with suspected ACS impose a considerable organizational burden to the ED, whilst overcrowding in this short stay unit has also been associated with ACS-induced posttraumatic stress disorder, which is now recognized as a major contributing factor for recurrence of ACS and overall mortality (14). Therefore, diagnostic algorithms allowing to make an early diagnosis or rule-out of ACS should be welcomed, since these would permit to help reducing overcrowding and concomitantly enhancing the possibility of obtaining more favourable clinical outcomes. Another important aspect emerged from our study is that the overall cost of patient management in the ED may also be reduced by replacing conventional CS-cTnI techniques with the novel HS-cTnI immunoassays. Considering that these novel methods are now commercialized at virtually the same price as the former CS-cTnI immunoassays, the overall financial saving approximated 20% in our study, potentially increasing to 33% and 46% using a 2-hour or 1-hour strategy for serial testing with HS-TnI. In another recent study, Kaambwa et al. carried out an economic analysis comparing the efficiency of HS-cTnT and CS-cTnT in terms of financial savings for adverse clinical outcome avoided (15), and concluded that the use of HS-cTnT had an incremental cost-effectiveness ratio.
The net economic benefit observed in our investigation was however partially counterbalanced by the higher rate of patients who would have been hospitalized for additional diagnostic investigations based on positive results of HS-cTnI (i.e., 9.4%). Whether these patients should be considered truly “false-positive”, or else they shall actually need a more aggressive monitoring and/or management remains a matter of debate (16,17). Nevertheless, recent evidence suggests that the risk of ACS is 60% higher in patients with cTnI values above the functional sensitivity of the HS immunoassay used in our investigation than in those with lower values (4). Therefore, although the hospitalization of these patients may contribute to increasing the overall hospital expenditure, it cannot be excluded that a more aggressive management may be advisable for preventing short-term recurrence and/or mortality. This is at least in part confirmed by the fact that one of the patients discharged with a negative result of CS-cTnI in our study (but with a positive result of HS-cTnI, blinded to the emergency physician at the time of ED admission) was then readmitted shortly thereafter with a diagnosis of ACS.
Conclusions
The results of this study suggest that replacing CS-cTnI with HS-cTnI immunoassays may be effective to enhance the efficiency of early ACS rule-out, but also generates a favourable organizational and economic impact in the ED.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was performed in accordance with the Declaration of Helsinki, under the terms of relevant local legislation. Since the HS-cTnI immunoassay was performed using residual material after routine CS-cTnI testing had been completed, and HS-cTnI test results were not reported to both the emergency physician and to the patient, informed consent was deemed unnecessary.
References
- Alvin MD, Jaffe AS, Ziegelstein RC, et al. Eliminating Creatine Kinase-Myocardial Band Testing in Suspected Acute Coronary Syndrome: A Value-Based Quality Improvement. JAMA Intern Med 2017. [Epub ahead of print]. [Crossref]
- Cervellin G, Lippi G. Of MIs and men--a historical perspective on the diagnostics of acute myocardial infarction. Semin Thromb Hemost 2014;40:535-43. [Crossref]
- Cervellin G, Mattiuzzi C, Bovo C, et al. Diagnostic algorithms for acute coronary syndrome-is one better than another? Ann Transl Med 2016;4:193. [Crossref]
- Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015;386:2481-8. [Crossref]
- Nestelberger T, Wildi K, Boeddinghaus J, et al. Characterization of the observe zone of the ESC 2015 high-sensitivity cardiac troponin 0h/1h-algorithm for the early diagnosis of acute myocardial infarction. Int J Cardiol 2016;207:238-45. [Crossref]
- Pickering JW, Than MP, Cullen L, et al. Rapid Rule-out of Acute Myocardial Infarction With a Single High-Sensitivity Cardiac Troponin T Measurement Below the Limit of Detection: A Collaborative Meta-analysis. Ann Intern Med 2017;166:715-24.
- Galli C, Lippi G. High-sensitivity cardiac troponin testing in routine practice: economic and organizational advantages. Ann Transl Med 2016;4:257. [Crossref]
- Lippi G, Dipalo M, Avanzini P, et al. Analytical assessment of the Beckman Coulter Unicel DxI AccuTnI+3 immunoassay. Diagnosis 2014;1:195-7. [Crossref]
- Krintus M, Kozinski M, Boudry P, et al. European multicenter analytical evaluation of the Abbott ARCHITECT STAT high sensitive troponin I immunoassay. Clin Chem Lab Med 2014;52:1657-65.
- Lippi G. Biomarkers: Novel troponin immunoassay for early ACS rule-out. Nat Rev Cardiol 2016;13:9-10. [Crossref]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref]
- Foley M, Kifaieh N, Mallon WK. Financial impact of emergency department crowding. West J Emerg Med 2011;12:192-7.
- Li WJ, Chen XM, Nie XY, et al. Early diagnostic and prognostic utility of high-sensitive troponin assays in acute myocardial infarction: a meta-analysis. Intern Med J 2015;45:748-56. [Crossref]
- Edmondson D, Shimbo D, Ye S, et al. The association of emergency department crowding during treatment for acute coronary syndrome with subsequent posttraumatic stress disorder symptoms. JAMA Intern Med 2013;173:472-4. [Crossref]
- Kaambwa B, Ratcliffe J, Horsfall M, et al. Cost effectiveness of high-sensitivity troponin compared to conventional troponin among patients presenting with undifferentiated chest pain: A trial based analysis. Int J Cardiol 2017;238:144-50. [Crossref]
- Lippi G, Montagnana M, Aloe R, et al. Highly sensitive troponin immunoassays: navigating between the scylla and charybdis. Adv Clin Chem 2012;58:1-29. [Crossref]
- Kozinski M, Krintus M, Kubica J, et al. High-sensitivity cardiac troponin assays: From improved analytical performance to enhanced risk stratification. Crit Rev Clin Lab Sci 2017;54:143-72. [Crossref]
Cite this article as: Lippi G, Bonfanti L, Dipalo M, Aloe R, Cervellin G. Clinical, organizational and economic analysis of high-sensitivity cardiac troponin testing in the emergency department. Ann Res Hosp 2017;1:44.


