
Dilemmas regarding the management of endometriosis-related infertility
Introduction: symptoms, diagnosis, treatment, rationale
A major challenge in assisted reproductive technology (ART) is the evaluation and management of female infertility (1). Endometriosis may reflect one of the underlying factors of infertility, affecting almost 5–10% of women of reproductive age. It stands as a chronic, estrogen-dependent disease, characterized by the intrusion and development of endometrial-like tissues, namely glands and stroma, outside the uterine cavity (2-4). The classification of this disease still remains controversial (5) and it mainly relies on the revised American Society for Reproductive Medicine (r-ASRM) system that was proposed in 1997 (6). The aforementioned prevalent classification system combines the assessment of the ectopic lesions in ovaries and peritoneum, regarding their abnormal morphology, number, size, or position, giving a total score. Based on the provided score, endometriosis could be described as “minimal”, “mild”, “moderate”, and “severe” or alternatively, it could correspond to stage I, II, III, IV respectively (5,6). The culprit of the reported disease could embody the blend of a wide pallet of genes (7) in conjunction with different environmental factors (8), immunological (3), and hormonal parameters (2), that are further delineated in next section. Despite the fact that some patients affected by endometriosis display no clinical symptoms, the majority of them mainly suffer from chronic pelvic pain, dysmenorrhea and dyspareunia and most importantly infertility (3,9).
In routine clinical practice, the presence of clinical symptoms may contribute to the initiative towards investigation of suspected endometriosis (2,9,10). Another step towards an accurate and prompt diagnosis of endometriosis includes physical examination of the pelvis and the abdomen, presenting with promising results (10). The aforementioned diagnostic procedures are correlated with the imaging techniques, namely transvaginal sonography, rectal endoscopic sonography and magnetic resonance imaging (MRI) (11), which are mainly employed for infiltrating lesions as well as for the detection of a possible ovarian endometrioma (10). However, the above approaches fail to reveal peritoneal endometriotic lesions (10,12). This limitation may lead clinicians to opt for an invasive approach, in order to thoroughly evaluate the inner organs (13). The ultimate diagnosis could be accomplished through the method of laparoscopy. Hence, European Society of Human Reproduction and Embryology (ESHRE) recommends the combination of laparoscopy and histological evaluation to be the gold standard of endometriosis’ diagnosis (9), even though they both appear to be invasive with inadequate sensitivity and specificity (12). However, as unexplained infertility appears to endorse undiagnosed endometriosis, clinicians should consider adopting laparoscopic surgery in patients with unexplained infertility and recurrent implantation failure (RIF) (14). Lacking symptoms, laparoscopy partially contributes to the diagnosis of minimal or mild forms of endometriosis (10). On the other hand, numerous cytokines and other non-invasive biomarkers, with great emphasis on cancer antigen 125 (CA-125), have been described as promising diagnostic tools (9). In conclusion, one may extrapolate on the challenging nature of endometriosis regarding an ultimate and successful diagnosis and the therapy that it entails (12).
The main principal of endometriosis’ treatment includes pain relief, fertility enhancement and improvement of the endometrial quality of women affected by endometriosis (2,9,10). Clinicians could either adopt medical therapy or surgical methods, or even their combination, accompanied with a pre- or post-operation medical treatment (9). Various studies reported that medical treatment, including hormonal therapies, oral contraceptives, gonadotropin-releasing hormone (GnRH) agonists or antagonists, and aromatase inhibitors, demonstrates remarkable results regarding diminishment of the pain-associated endometriosis (15-17). In the context of surgical approaches, the option regarding which method to perform is mainly based on the ectopic lesions’ number, size, and position, being either apparent or infiltrated (18). Laparoscopy surgery is commonly employed in order to remove detectable endometriotic lesions in the organs and repair the affected pelvic anatomy or any abnormality (9,13). Combining medical and surgical therapy seems to result in slightly improved outcomes and pain relief in comparison to being applied separately (18). Nevertheless, a debate still exists in literature on whether or not to perform laparoscopy surgery for endometriosis-related infertility. The invasive nature of surgery and the subsequent complications, as well as the calibration of different surgical techniques, such as CO2 laser ablation, excision or cystectomy, aspiration via ultrasound, and drainage of lesions following laser vaporization (19,20), seem to encourage this conflict (9,21). Indicatively, the most commonly encountered surgical complication, especially in cases of cystectomy for ovarian endometriomas, is reported to be the reduced ovarian reserve and as a consequence the appearance of iatrogenic infertility or premature menopause (9). On the other hand, performing laparoscopy for treatment of early stages endometriosis exhibits promising results in refining fertility based on evidence published in literature (21). The aim behind surgical removal of peritoneal endometriotic implants is supposed to be limitation of pelvic inflammation, though this is still considered vague (20). The key recommendation in the hope to resolve the aforementioned conflict could be that clinicians should thoroughly ponder on the true impact of a possible endometriosis’ diagnosis in female infertility (10).
This perspective article aims to present the controversial issues challenging current clinical practice, regarding the management of endometriosis related to infertility. Numerous approaches are employed regarding endometriosis’ treatment. However, laparoscopy surgery still depicts the gold standard for either diagnosis or treatment of endometriosis-related infertility (20). The absence of a standardized protocol has led to questions and debates evident in the literature, arguing the benefits of laparoscopy versus disadvantages, especially in cases of unexplained infertility. Failing to concur on an optimal treatment leads the researchers towards elucidating the molecular mechanisms of the disease. Formulating an efficient management through a universally employed protocol buttressed by the delineation of the molecular mechanisms involved seems to be the holy grail of endometriosis-related infertility research.
Endometriosis’ management and challenges regarding in vitro fertilization (IVF) patients
Examining endometriosis’ management under the prism of infertility is the primary goal. It still remains indefinable whether the surgical approach with ablation of ectopic endometrial implants in peritoneum favors the pregnancy outcome, in an attempt to avoid IVF treatment. Most IVF patients with the underlying factor of endometriosis, report a certain array of events in their reproductive history. The combination of endometriosis’ idiopathic nature and the lack of clinical signs may delay the detection of the disease, particularly in patients with unexplained infertility who followed IVF treatment after failure to conceive following 2 years of attempts (22).
According to ESHRE guidelines in 2014, infertile patients with endometriosis should be advised towards IVF postoperatively, especially if tubal or male factor are involved (9). On the other hand, when referring to ART, the reappearance of endometriosis due to the employment of controlled ovarian stimulation (COS) protocols is a risk, concerning that endometriosis is an estrogen-dependent disease (23).
A retrospective study by Lee et al. indicated a significant natural conception rate in women presenting with endometriosis as the only reported infertility factor, during the first year following laparoscopy surgery (24). These findings are indicators that surgery potentially enhances natural conception and should probably be adopted in cases of women who wish to conceive naturally (2,20). The distinct group of patients presenting with unexplained infertility is most commonly accompanied with repeated embryo implantation failures, a condition known as RIF (14). Recent studies have voiced a relation between RIF and endometriosis, adding another level of complexity with respect to either diagnosis or treatment of endometriosis (9). It may be hypothesized that, the management of IVF patients with unexplained infertility and RIF, may result in IVF overuse, which could be potentially avoided by laparoscopic correction of endometriosis followed by natural conception (22). Assuming that laparoscopy improves endometriosis in a group of patients with unexplained infertility and RIF, it also seems to restore their fecundity and to alleviate the psychological burden resulting from the consecutive failed attempts to conceive. The above are supported by two previous randomized controlled trials, of which the first had observed encouraging pregnancy rates in women affected with endometriosis following endometrial lesions diminishment by laparoscopy (25), while the other study found similar pregnancy rates between the treated group and the no-treated control group (26).
The element of futile fertility treatments and possible overuse of IVF in patients presenting with unexplained infertility yet harboring undiagnosed endometriosis should not be overlooked. Concurring on the optimal time frame that laparoscopy should be suggested to this strictly defined cohort of patients is a mission undertaken by several research groups focusing on endometriosis-related infertility. Failing to timely diagnose endometriosis to these patients and instead treat them under the umbrella of unexplained infertility is the ultimate risk. This may harbor the peril of investing time and efforts in failed treatments that may ultimately cost them their most dynamic and valuable reproductive years. In addition, the financial and psychological strain associated with IVF overuse has been thoroughly addressed in literature and is a component that merits further investigation.
Mechanisms of endometriosis pathophysiology related to infertility
The research of the underlying molecular mechanisms behind endometriosis is considered at this point to be of a paramount importance as it is assumed that all the controversial issues regarding endometriosis’ management are directly related to the vague etiology of this disease. In general, the pathophysiology of endometriosis still remains obscure, since both genetic and environmental parameters are implicated (8,12). Hence, it may be dependent on numerous genes, such as the chromosomal loci on genes WNT4, VEZT and near GREB1 (7) and thus being inherited or even influenced by women’s race (8). Moreover, the contribution of other risk factors has been reported, such as age, lifestyle, smoking with the oxidative stress it entails, various toxins, autoimmunity, as well as existing inflammation in lower genital tract (2,12,27).
Various theories have been proposed and presented in literature in an effort to decrypt the endometriosis’ pathogenesis. The most established and complete theory is retrograde menstruation (28). According to this theory, several endometrial products and cell debris of the menstrual fluid enter the fallopian tubes inversely, towards peritoneum, where they implant and commence to grow (2,3,27). It is further suggested that some endometrial epithelial and mesenchymal stem cells may implant during retrograde menstruation (2). Some theories refer to Müllerian remnant abnormalities, while others address ovarian endometriosis, suggesting that coelomic epithelium covering peritoneal tissues and gonads transforms into endometrium-like tissues (2,3,29).
The role of the steroid hormone Estradiol (E2) during menstrual cycle is to restore endometrium, which is normally degraded under the influence of progesterone. Endometrial E2 levels originate from the ovaries and the adrenal glands, which produce and secrete E2 hormone into circulation (3). In patients suffering from endometriosis, it has been observed that both eutopic and ectopic endometriotic tissues demonstrate an enhanced local E2 production, resulting to the endorsement of proliferation and implantation of ectopic lesions (3,29). This phenomenon is attributed to the high concentration of the enzyme aromatase P450, which participates in the conversion of androstenedione to estrone, and it is observed to be additionally expressed by endometriotic lesions (3), in combination with decreased levels of the enzyme 17β-hydroxysteroid dehydrogenase type 2, which in turn catalyzes the reaction of estradiol into estrone (29). In addition to this different hormonal biosynthesis, it has been detected that the levels of isoforms of estrogen receptors α and β (ER-α and ER-β respectively) are notably affected, as a result of the incomplete methylation of promoter of ER-β gene, leading to an unusual up-regulation of ER-β expression in conjunction with a down-regulation of ER-α expression (2).
On the same note, ectopic endometriotic lesions present with either lower expression or even dysfunctional biosynthesis of isoforms of progesterone receptors α and β (PR-α and PR-β) (3,29). Hence, a resistance to progesterone is developed, being another brick in the wall of endometriosis pathogenesis. Progesterone is widely known as another key steroid hormone produced during luteal phase of the menstrual cycle. It plays a vital role in the process of endometrial decidualization and prepares the base for a possible embryo implantation (2,3). The consequence of progesterone resistance in endometriotic lesions is reported to be an altered expression of specific genes involved in the procedure of decidualization and embryo implantation (2), through modifications of acetylation and deacetylation. Typical representative proteins expressed by these modified genes could be the Insulin-like growth factor 1 (IGF-1), prolactin, glycodelin, cell cycle regulators such as cyclins and the transcription factors associated with targets Homeobox A10 (HOXA10) and forkhead box O1 (FOXO1) (3,30).
Similar to the process of decidualization, endometriotic lesions need the support of the vascular network, which appears to be crucial for their maintenance and further expansion (3,30). Hence, certain angiogenic factors are detected in increased levels in the peritoneal fluid, in conjunction with the development of new blood vessels, resulting from formerly prevailing vessels and/or de novo, through the procedure of angiogenesis and vascularization respectively (3). For this reason, several angiogenesis-related factors including vascular endothelial growth factor (VEGF), angiopoietins (ANGPTS and Tie 2), platelet-derived growth factor (PDGF)-BB and transforming growth factor beta 1 (TGF-β1) seem to be up-regulated in patients with endometriosis (30), along with the vascularization-related factors, namely the bone marrow-derived endothelial progenitor cells and the stromal cell-derived factor 1. The later may intervene to the vessels’ formation, assisted by specific angiogenetic cytokines, such as bone morphogenic protein 2 (BMP2), interleukin 6 and 8 (IL-6 and IL-8) and tumor necrosis factor-α (TNF-α). Finally, studies demonstrate that VEGF production is further promoted by E2 levels that appear to be locally elevated in endometriosis (3).
The invasion of ectopic endometriotic lesions into the peritoneal cavity is enough to trigger inflammation as a response of patients’ immune system, however, it is remarkable how these endometrial cells finally manage to survive and avoid apoptosis (2,29). Indeed, studies reveal that women with endometriosis demonstrate an altered phenotype regarding the “fate” of these endometrial cells, including an amplified expression of anti-apoptotic factors, which consequently inhibits the delicate pathway of apoptosis of endometrial cells (29). A recent study documented higher levels of the apoptosis’ inhibitors of H-linked inhibitor of apoptosis (XIAP) and heat shock protein 27 (HSP27) in women who were diagnosed with endometriosis. Assuming that the aforementioned factors are increased during the “receptivity window”, this may entail poor endometrial receptivity and thus contribute to an embryo’s failure to implant into the uterine cavity (31). It is important to acknowledge that inflammatory response is a responsible factor resulting to poor oocyte quality with subsequent reduced fertilization and implantation rate (21,32).
Another possible etiology of endometriosis’ pathogenesis appears to be the defective immune response, with a subsequent overexpression of various prostaglandins and chemokines, especially Regulated on Activation, Normal T-cell Expressed and Secreted (RANTES), monocyte chemoattractant protein-1 (MCP-1) and IL-8 (2). In addition, studies recorded that the function of natural killer cells is suppressed, while macrophages and leukocytes are on alert, where the menstrual debris fail to be removed and thus the endometrial cells are favored to proliferate (29). Regarding the impact of macrophages on endometriosis’ pathogenesis, it is known that they are efficient in incorporating and recycling iron that originates from the breakdown of erythrocytes, especially in cases of endometriosis which entails inordinate amounts of pelvic blood (2). Within this toxic environment, compromised by iron accumulation and reactive oxygen species production, both endometriosis and its symptoms seem to persist or even progress (29).
In an effort to delineate the chaotic nature of the genetic mechanism (27), a bioinformatics analysis of microarray data was employed. Ping et al. attempted to detect which genes could be connected to endometriosis’ pathogenesis in the presence of an altered expression. The analysis dictated an up-regulation of genes related to focal adhesion, regulation of actin cytoskeleton, MAPK signaling pathway and TGFB/SMAD signaling pathway (4). The results mentioned are in accordance to another network analysis, which indicates the dysregulation of these pathways, with several signal transducers and activators of transcription (STATs) to be further involved (12). In the first scale, disorder of extracellular matrix and cytoskeleton communication mainly due to differentially expressed gene of extracellular element fibronectin 1 (FN1) or in combination with these of epidermal growth factor (EGF) and epidermal growth factor receptor (EGFR), is proposed to promote the progress of endometriosis. This appears to be in conjunction with different development of cell adhesions related to actin skeleton in the endometrial cavity owning to alteration of genes FN1, EGF, EGFR, Ras-related C3 botulinum toxin substrate 1 (RAC1). Furthermore, overexpression of genes of EGF, EGFR, TGFB1 and RAC1 seems to stimulate the MAPK signaling pathway, with the contribution of MEK/ERK proteins to MYC proto-oncogenes encoding for endometrial cell proliferation. Last but not least, activation of TGFB pathway is reported to be involved in pathogenesis of the disease, primarily due to its intervention to signaling in the epithelial and stromal cells of endometrium (4,12).
All the aforementioned endometriosis’s pathogenic mechanisms that encompass several molecular pathways may directly be associated with infertility. Indeed, endometriosis and infertility could be likening communicating vessels, with endometriosis including abnormalities in pelvic anatomy, alteration of hormonal levels, declined implantation, and reduced oocyte quantity or quality (10).
Conclusions
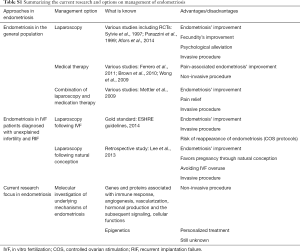
This article focuses on presenting perspectives contributing to illuminating the conflict of management regarding endometriosis-related infertility. A relevant table regarding the summary of current research and options on endometriosis’ management is provided (Table S1), along with a second table reporting on the consecutive steps entailed from endometriosis’ pathogenesis to treatment (Table S2). The existing diagnostic approaches regarding endometriosis remain invasive and the therapeutic benefits fail to be guaranteed, while the treatment mainly alleviates the clinical signs of the disease. Laparoscopy is mostly employed for diagnosis and correction of endometriosis, potentially enabling positive natural conception pregnancy rates, which may contribute towards avoiding IVF overuse, especially in cases of patients with unexplained infertility and RIF. For these reasons, the scientific interest turns to decoding the molecular mechanisms of pathophysiology of this disease, which still appear to be obscure. A potential advantage of this trend could be formulation of personalized treatment that relies on several genes’ expression and/or specific signaling pathways. However, an approach of the actual molecular mechanisms underlying the endometriosis’ pathogenesis is difficult to be established, as different groups of genes and proteins appear to be implicated, associated with immune response or function, angiogenesis and vascularization, hormonal production and the subsequent signaling, cellular mechanisms and more. It should be highlighted that fueling the hope of delineating and decoding the complex molecular physiology of endometriosis lies in our aspiration to preclude the possibility of performing laparoscopy based on suspicions of false evidence. All the risks that are entailed in employing an invasive technique towards endometriosis’ diagnosis could be made redundant if the spotlight of medical research focused further on the molecular investigation of this perplexing condition, making treatment individualized and effective.
Full table

Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Abrao MS, Muzii L, Marana R. Anatomical causes of female infertility and their management. Int J Gynaecol Obstet 2013;123:S18-24. [Crossref] [PubMed]
- Vercellini P, Viganò P, Somigliana E, et al. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261-75. [Crossref] [PubMed]
- Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and Immune Dysfunction in Endometriosis. BioMed Res Int 2015;2015:795976. [PubMed]
- Ping S, Ma C, Liu P, et al. Molecular mechanisms underlying endometriosis pathogenesis revealed by bioinformatics analysis of microarray data. Arch Gynecol Obstet 2016;293:797-804. [Crossref] [PubMed]
- Johnson NP, Hummelshoj L, Adamson GD, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod 2017;32:315-24. [Crossref] [PubMed]
- American Society for Reproductive. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817-21. [Crossref] [PubMed]
- Nyholt DR, Low SK, Anderson CA, et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet 2012;44:1355-9. [Crossref] [PubMed]
- Dai Y, Li X, Shi J, et al. A review of the risk factors, genetics and treatment of endometriosis in Chinese women: a comparative update. Reprod Health 2018;15:82. [Crossref] [PubMed]
- Dunselman GAJ, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014;29:400-12. [Crossref] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012;98:591-8. [Crossref] [PubMed]
- Bazot M, Lafont C, Rouzier R, et al. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril 2009;92:1825-33. [Crossref] [PubMed]
- Aznaurova YB, Zhumataev MB, Roberts TK, et al. Molecular aspects of development and regulation of endometriosis. Reprod Biol Endocrinol 2014;12:50. [Crossref] [PubMed]
- Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev 2014.CD011031. [PubMed]
- Coughlan C, Ledger W, Wang Q, et al. Recurrent implantation failure: definition and management. Reprod Biomed Online 2014;28:14-38. [Crossref] [PubMed]
- Ferrero S, Gillott DJ, Venturini PL, et al. Use of aromatase inhibitors to treat endometriosis-related pain symptoms: a systematic review. Reprod Biol Endocrinol 2011;9:89. [Crossref] [PubMed]
- Brown J, Pan A, Hart RJ. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst Rev 2010.CD008475. [PubMed]
- Wong CL, Farquhar C, Roberts H, et al. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst Rev 2009.CD002120. [PubMed]
- Mettler L, Ruprai R, Alkatout I. Impact of medical and surgical treatment of endometriosis on the cure of endometriosis and pain. Biomed Res Int 2014;2014:264653. [Crossref] [PubMed]
- Karaman Y, Uslu H. Complications and their management in endometriosis surgery. Womens Health (Lond) 2015;11:685-92. [Crossref] [PubMed]
- Berlanda N, Vercellini P, Somigliana E, et al. Role of Surgery in Endometriosis-Associated Subfertility. Semin Reprod Med 2013;31:133-43. [Crossref] [PubMed]
- Afors K, Murtada R, Centini G, et al. Employing Laparoscopic Surgery for Endometriosis. Womens Health (Lond) 2014;10:431-43. [Crossref] [PubMed]
- Kamphuis EI, Bhattacharya S, van der Veen F, et al. Are we overusing IVF? BMJ 2014;348:g252. [Crossref] [PubMed]
- Polat M, Yaralı İ, Boynukalın K, et al. In vitro fertilization for endometriosis-associated infertility. Womens Health (Lond) 2015;11:633-41. [Crossref] [PubMed]
- Lee HJ, Lee JE, Ku SY, et al. Natural conception rate following laparoscopic surgery in infertile women with endometriosis. Clin Exp Reprod Med 2013;40:29. [Crossref] [PubMed]
- Marcoux S, Maheux R, Bérubé S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med 1997;337:217-22. [Crossref] [PubMed]
- Parazzini F. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Gruppo Italiano per lo Studio dell'Endometriosi. Hum Reprod 1999;14:1332-4. [Crossref] [PubMed]
- Kobayashi H, Uekuri C, Shigetomi H. Towards an understanding of the molecular mechanism of endometriosis: unbalancing epithelial-stromal genetic conflict. Gynecol Endocrinol 2014;30:7-15. [Crossref] [PubMed]
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927;14:422-69. [Crossref]
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med 2014;2014:179515. [Crossref] [PubMed]
- Kobayashi H, Iwai K, Niiro E, et al. Fetal programming theory: Implication for the understanding of endometriosis. Hum Immunol 2014;75:208-17. [Crossref] [PubMed]
- Antsiferova YS, Sotnikova NY, Bogatova IK, et al. Changes of apoptosis regulation in the endometrium of infertile women with tubal factor and endometriosis undergoing in vitro fertilization treatment. JBRA Assisted Reproduction 2014;18:2-6. [Crossref]
- Cakmak H, Taylor HS. Molecular mechanisms of treatment resistance in endometriosis: the role of progesterone-hox gene interactions. Semin Reprod Med 2010;28:69-74. [Crossref] [PubMed]
Cite this article as: Simopoulou M, Sfakianoudis K, Tsioulou P, Rapani A, Pantos K, Koutsilieris M. Dilemmas regarding the management of endometriosis-related infertility. Ann Res Hosp 2019;3:6.


